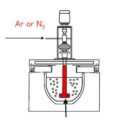
Sound metal is used to refer to the quality of the molten metal immediately before casting, which means the removal of dissolved hydrogen and non-metallic impurities from the molten metal.
When the metal solidifies, the solubility of hydrogen in the aluminum alloy decreases by about an order of magnitude. Therefore, if the hydrogen content of the molten metal does not fall below the solid solubility limit of hydrogen in the metal, hydrogen will be released from the metal during the casting process. Hydrogen can create pinholes in rapidly solidifying metals (such as direct cooling ingots) or fill shrinkage cavities in slowly solidifying metals. The hydrogen remaining in the metal even after solidification is harmful because it diffuses into the voids and other discontinuities in the solid metal during the heat treatment process, thereby exacerbating the harmful effects of these defects on the metal properties. Excess hydrogen can cause bright flakes in forgings and bubbles in rolled products.

Molten aluminum before casting contains many impurities. If these impurities are not removed, a large amount of waste material will be lost during casting, or the metal quality of the products made from it will be poor. In molten aluminum-based alloys, the main harmful impurities are dissolved hydrogen and suspended non-metallic particles, such as aluminum and magnesium oxides, refractory particles, etc. Since aluminum is a highly oxidizing metal, aluminum oxide is generated during the process of melting aluminum in molten aluminum. When the amount of alumina produced increases, the recovery rate of aluminum decreases. In addition, paint and other inclusions are usually contained in the aluminum block and added to the molten aluminum. As the amount of such inclusions increases, the purity of aluminum decreases.
In order to get the sound metal and solve the problems caused by such alumina and inclusions, molten aluminum is treated with a flux that can prevent aluminum from oxidizing and can trap the inclusions.
In the past, reducing the dissolved gas content and non-metallic impurity content of molten metal was achieved by keeping the temperature in furnaces and other metal processing vessels as low as possible, and keeping the metal in the molten liquid state for a long time. However, this time-consuming practice has been modified and largely replaced by various flux methods in which molten metal is in contact with reactive gases or solid fluxes that usually contain halogens. The most commonly used flux in aluminum processing is chlorine or chlorine generating compounds, such as hexachloroethane. In most alloy grades, chlorine flux can satisfactorily remove hydrogen and non-metallic impurities.