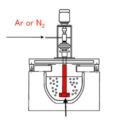
Pressure difference degassing refining method can be divided into gas degassing method, flux degassing method, vacuum degassing method and so on.
The partial pressure difference degassing refining method places the gas-dissolved metal in a vacuum with a small hydrogen partial pressure, or introduces an inert gas into the melt to provide the driving force for dehydrogenation. In industrial production, inert gas such as N2 is usually passed into the melt, or a gas-generating flux is pressed into the melt inside the bubble. There is no hydrogen at all at first, that is, the partial pressure of hydrogen is zero, and the partial pressure of hydrogen in the melt around the bubble is greater than or equal to zero. Under the effect of the internal and external hydrogen partial pressure difference, the dissolved hydrogen atoms diffuse to the melt bubble interface, where they recombine into hydrogen molecules into the bubble, and then the hydrogen molecules float up with the bubble to the melt surface and escape. This process will continue until the partial pressure of hydrogen inside and outside the bubble is equal, that is, in a state of equilibrium.

The partial pressure difference degassing refining method can be divided into gas degassing method, flux degassing method, vacuum degassing method and other gas degassing methods. The gas used in gas degassing method includes inert gas, active gas and mixed gas. In addition, there are gas degassing methods in which solid flux powder is added to the refined gas and flux mixture degassing methods.
Inert gas refining method. Inert gas refers to a gas that does not chemically react with the melt and the gas dissolved in the melt, and it does not dissolve in the melt. Such gases include argon, ammonia, and nitrogen, which can also be considered indifferent. gas. Because nitrogen rarely reacts with aluminum at 800°C, it is not soluble in molten aluminum. Due to the high price of argon and ammonia, the technology of equipment recovery and purification equipment is complicated, and most of them are not used. Nitrogen is mainly used in production.