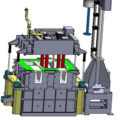
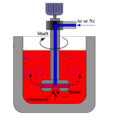
Online rotary degassing is necessary for all molten metal production.
At the aluminum melting temperature (700°C and above), water vapor decomposes into hydrogen and oxygen. Oxygen reacts with the aluminum liquid to form alumina, and the hydrogen (H) decomposes into hydrogen atoms (H) and dissolves into the aluminum liquid. Therefore, it is inevitable to dissolve hydrogen in molten aluminum, and how much it dissolves depends on the humidity of the air. Then, since the solubility of hydrogen in molten aluminum is much greater than that in solid aluminum, pores will be formed in the crystal grain boundaries during the steep drop of the solidification temperature of the dissolved hydrogen. The pores in the metal can harm the strength of the metal. In order to increase the strength of the metal, it is necessary to consider removing most of the gas when the metal is liquid.

The rotary degassing method uses a high-speed rotating impeller to transform the purified gas into small and evenly distributed bubbles. These small gas slowly rise to the liquid surface, which lengthens the reaction time.
A faster degassing rate can be obtained by online rotating degassing technology. The graphite shaft and the impeller head are immersed in the molten pool, and the large bubbles are cut into small, uniform bubbles.
The reduction in the size of the bubbles of the purified gas significantly increases the surface area and residence time of the purified gas. The stirring effect of the impeller head makes the purified gas more evenly distributed, so that the purified gas can fully contact the hydrogen in the aluminum liquid, which is beneficial Remove hydrogen.
Although the online rotary degassing method is effective in improving the efficiency of hydrogen removal, it is difficult to determine its role in removing oxides. Studies have shown that the small bubbles of argon or nitrogen generated in the rotating degassing method can make the oxide float to the liquid surface.
If a small amount of chlorine is added to the purified gas, the efficiency of removing oxides can be improved. Since chlorine can change the surface tension of the molten aluminum, it is convenient for oxides to adhere to the bubbles of the purified gas.
The rotating degassing method can increase the amount of slag on the surface of the molten aluminum, part of which is due to the inclusions and non-metallic particles in the molten aluminum floating to the surface.