Metals degassing is used to treat molten metal to effectively remove undesirable inclusions such as gases, alkali metals, and entrained solids.
When many molten metals are used in casting and similar processes, they must be initially treated to remove harmful components that may adversely affect the physical or chemical properties of the resulting cast product. For example, molten aluminum and aluminum alloys from alumina reduction tanks or metal holding furnaces usually contain dissolved hydrogen, solid non-metallic inclusions (aluminum carbides) and various reactive elements (such as alkali metals and alkaline earth metals). As the metal cools, the dissolved hydrogen escapes from the solution and forms harmful pores in the product. Non-metallic solid inclusions can reduce metal cleanliness, while reactive elements and inclusions can produce harmful metallic properties.

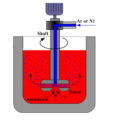
These undesirable components are usually removed from molten metal by introducing gas below the surface of the metal by means of a gas injector. As the generated bubbles rise through the molten metal mass, the bubbles absorb the gas dissolved in the metal and remove it from the melt. In addition, the non-metallic solid particles are swept to the surface by the flotation effect of bubbles and can be skimmed off. If the gas used for this purpose reacts with the contained metal impurities, the element can be converted into a compound by a chemical reaction and removed from the melt in the same way as the contained solid or by liquid-liquid separation.
This process is usually called “metals degassing”, and it can be used not only for metal degassing but also for inclusions removal. The process is usually carried out in one of two ways: in the furnace, usually one or more static gas injection pipes are used; or in series, by passing the metal through the box in a tank usually placed between the holding furnace and the casting machine In the exhaust pipe, a more effective gas injector can be used. In the first case, the process is inefficient and time-consuming because a large number of bubbles are generated, which can lead to poor gas/metal contact, poor metal agitation, and high surface turbulence and splashing. The resulting surface turbulence can cause ash formation and metal loss, and poor metal agitation can lead to some untreated metals. The second method is more effective in introducing and using gas. Part of the reason is that the online method is a continuous process rather than a batch process.
In order for the online processing to be carried out effectively, the bubbles must be in contact with the melt for an appropriate period of time, which is achieved by injecting inert gas. For example, the gas is broken down into smaller bubbles and the smaller bubbles by rotating degasser, which is more effectively dispersed by the volume of metal. Effectiveness is usually defined in terms of the hydrogen degassing reaction of aluminum and an adequate reaction is generally considered to remove at least 50% of the hydrogen (usually 50% to 60%).