Aluminum alloy is easy to inhale and oxidize in the process of casting, so there are gas and various non-metallic inclusions in the melt, which makes the ingot loose, porosity, inclusion and other defects, significantly reduces the mechanical properties, processing properties, fatigue resistance, corrosion resistance, anodic oxidation resistance and other properties of aluminum, and even causes the products to be scrapped. In addition, due to the influence of raw materials and auxiliary materials, there may be some other metals harmful to the melt, such as Na, Ca and alkaline earth metals. Some alkali metals have adverse effects on the properties of most aluminum alloys. For example, sodium in a-mg alloys with high magnesium content not only causes “sodium brittleness”, but also reduces the melt fluidity and affects the casting properties of the alloys. Therefore, special degassing process in aluminium should be taken to remove gas, non-metallic inclusions and other harmful metals in aluminum alloy to ensure product quality.

The requirements of aluminum and aluminum alloy for melt purification vary according to different material uses. Generally speaking, for products with general requirements, the hydrogen content should be controlled below 0.15-0.2ml / (100ga), and the single particle of non-metallic inclusion should be less than 10um; for aviation materials with special requirements, the hydrogen content of double zero foil should be controlled below 0.1ml / (100ga), The single particle size of nonmetallic impurity should be less than 5um. Of course, due to different detection methods, the measured hydrogen content will be different. Generally, nonmetallic inclusions are detected qualitatively by ingot macrostructure and ultrasonic flaw detection of aluminum materials, or quantitatively detected by slag detector.
Degassing Process In Aluminium
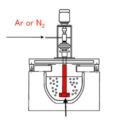
There are three main degassing methods: partial pressure degassing, pre solidification degassing and vibration degassing.
Partial pressure degassing principle uses the principle of the influence of gas partial pressure on the gas solubility in the melt to control the partial pressure of hydrogen in the gas phase, resulting in a large partial pressure difference between the hydrogen partial pressure in equilibrium with the dissolved gas in the melt and the hydrogen partial pressure of the actual gas, which produces a large dehydrogenation driving force and makes the hydrogen quickly eliminated. If pure inert gas is introduced into the melt, or the melt is placed in vacuum, because the partial pressure of the initial inert gas and hydrogen in vacuum is p≈ 0, while the equilibrium partial pressure P of dissolved hydrogen in the melt is far greater than 0, there is a large partial pressure difference between the bubbles of the melt and the inert gas and between the melt and the vacuum,
In this way, the hydrogen in the melt will quickly diffuse into the bubble or vacuum, enter the bubble or vacuum, and compound into molecular state to eliminate. This process continues until the hydrogen partial pressure in the bubble is equal to the equilibrium partial pressure of hydrogen in the melt, that is, it is in a new equilibrium state.