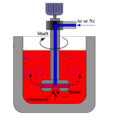
The molten aluminum degassing with nitrogen is usually introduced into the melt through a rotary degassing device. This degassing process is limited by the laws of thermodynamics; when purge bubbles are introduced into the melt, they float towards the surface, thereby collecting hydrogen. In the best case, these hydrogen-saturated bubbles leave the melt and reduce the hydrogen content. In this case, from a thermodynamic point of view, the processing efficiency is 100%. However, as the gas content in the melt decreases, the equilibrium pressure of hydrogen in the bubbles will also decrease, so the amount of purge gas required to remove residual hydrogen must be increased.
The equilibrium gas removal rate of pure aluminum higher than 1,400F. For example, a gas removal ratio of 200 means that 200 liters of inert gas are required to remove one liter of hydrogen. This behavior limits the ability of metal foundries to degas hydrogen to extremely low levels. The solubility also increases exponentially with temperature, which means that an increase of 200F doubles the solubility. Under all the same conditions, a higher aluminum melt temperature will increase the necessary degassing time.

Alloying elements also affect the solubility of hydrogen. The role of alloying elements is characterized by changes in alloy correction coefficients. Table 1 lists some common casting alloys. Alloys with larger values are more difficult to degas. Fortunately, in most cases, these factors can be controlled, and the gas content and process required to eliminate excessive pores in aluminum castings can be achieved without undue difficulties.
In the process of purifying nitrogen, the aluminum is degassed by improving the rotating nozzle, which increases the contact area between nitrogen and aluminum, thereby improving the effect of degassing and removing slag, and achieves the purpose of aluminum purification. improve product quality.
The key to aluminum degassing with nitrogen is that the rotor can decompose the incoming nitrogen bubbles into small bubbles and spread them throughout the molten metal. By reducing the bubble diameter, the surface area of nitrogen increases sharply, making more nitrogen surface contact with hydrogen and impurities in the molten metal, and as the bubbles rise, hydrogen or impurities are removed from the molten aluminum.
The flow control of the entering aluminum nitrogen can adjust the gas flow according to the volume of the metal liquid to be processed, and the speed of the rotating rod and the rotor can be adjusted to generate bubbles of appropriate size to facilitate the diffusion of inert gas.